Rubiacin
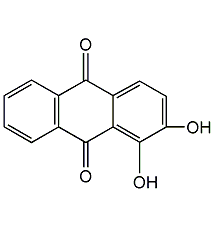
Structural formula
| Business number | 01H2 |
|---|---|
| Molecular formula | C14H8O4 |
| Molecular weight | 240.21 |
| label |
1,2-dihydroxy-9,10-anthracenedione, 1,2-Hydroxyanthraquinone, Alizarin, 1,2-dihydroxy, 1,2-dihydroxyricinone, 1,2-Dihydroxyanthraquinone, 1,2-Dihydroxy-9,10-anthracenedione, Mordant Red 11, Pigment red 83, Anti-inflammatory |
Numbering system
CAS number:72-48-0
MDL number:MFCD00001201
EINECS number:200-782-5
RTECS number:CB6580000
BRN number:1914037
PubChem number:24845856
Physical property data
1. Properties: orange-red crystal or ocher yellow powder
2. Density (g/mL, 20/4℃): 1.06
3. Relative vapor density (g /mL, air=1): Uncertain
4. Melting point (ºC): 289.5
5. Boiling point (ºC, normal pressure): 430SU
6. Boiling point (ºC, 5.2 kPa): Uncertain
7. Refractive index: Uncertain
8. Flash point (ºC): 430 (subl.)
9. Specific optical rotation (º): Uncertain
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25 ºC): Uncertain
12. Saturation vapor pressure (kPa, 60 ºC): Uncertain
13. Heat of combustion (KJ/mol) : Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Oil and water (octanol /water) distribution coefficient: Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18. Explosion lower limit (%, V/V) : Uncertain
19. Solubility: Easily soluble in hot methanol and ether at 25°C. Soluble in benzene, glacial acetic acid, pyridine, carbon disulfide, slightly soluble in water
Toxicological data
Acute toxicity: oral LD50 of wild birds: 316 mg/kg; mutagenicity: Salmonella Mutation in microorganismsTEST SYSTEM: 100 ug/plate; Bacillus subtilis DNA repairTEST SYSTEM: 2 mg/disc; Rat liver Unscheduled DNA synthesisTEST SYSTEM: 10 mg /L; Salmonella Mutation in microorganismsTEST SYSTEM: 100 ug/plate;
Ecological data
None
Molecular structure data
1. Molar refractive index: 62.43
2. Molar volume (cm3/mol): 155.9
3. Isotonic specific volume (90.2K ): 465.2
4. Surface tension (dyne/cm): 79.2
5. Polarizability (10-24cm3): 24.74
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 16
6. Topological molecule polar surface area 74.6
7. Number of heavy atoms: 18
8. Surface charge: 0
9. Complexity: 378
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stable properties under normal temperature and pressure.
2. It is irritating. When using, avoid inhaling the dust of this product and avoid contact with eyes and skin.
Storage method
1. Seal and store.
Synthesis method
1. In nature, alizarin is found in the roots of madder. Melt anthraquinone-2-sulfonic acid with caustic soda and potassium chlorate or potassium nitrate, then pour the melt into hot water, and then use hydrochloric acid to precipitate alizarin: 9g potassium chlorate, 30g anthraquinone-2-sulfonate Dissolve sodium acid and 110g of sodium hydroxide in 110ml of water, and heat and react 25g in an autoclave at 170°C. After cooling, the reaction product was extracted with water several times, 150 ml each time. The water extract was filtered, and the filtrate was acidified with hydrochloric acid. After cooling, the precipitated precipitate is suction-filtered, washed, and dried to obtain about 20g of alizarin.
Purpose
1. Acid-base indicator. Used as drip reagent for aluminum, indium, mercury, zinc and zirconium. Stain for in vivo staining of nervous tissue and protozoa.
2.Rubicin has an inhibitory effect on the growth of Staphylococcus aureus and can inhibit the permeability of rat skin connective tissue. It is similar to rutin. Similar, may have anti-inflammatory effects. It can be safely used as cosmetic coloring and lipstick coloring without any side effects. Combined with some phenolic hydroxyl groups and aniline derivatives, it can be used as a non-irritating oxidative hair dye additive to make the color soft and lasting.
extended-reading:https://www.bdmaee.net/dabco-t-12-tin-catalyst-nt-cat-t-120-dabco-t-12/extended-reading:https://www.cyclohexylamine.net/dioctyldichlorotin-dichlorodi-n-octylstannane/extended-reading:https://www.newtopchem.com/archives/1053extended-reading:https://www.cyclohexylamine.net/catalyst-1027-polyurethane-catalyst-1027/extended-reading:https://www.newtopchem.com/archives/category/products/page/27extended-reading:https://www.newtopchem.com/archives/category/products/page/153extended-reading:https://www.cyclohexylamine.net/nn-dicyclohexylmethylamine-2/extended-reading:https://www.bdmaee.net/nt-cat-e-129-elastomer-catalyst-elastomer-catalyst-nt-cat-e-129/extended-reading:https://www.cyclohexylamine.net/tetramethyl-13-diaminopropane-tmeda/extended-reading:https://www.bdmaee.net/dabco-eg-catalyst-cas280-57-9-evonik-germany/


