1-Aminoanthraquinone
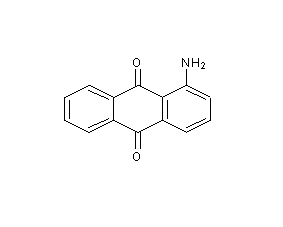
Structural formula
| Business number | 01SY |
|---|---|
| Molecular formula | C14H9NO2 |
| Molecular weight | 223.23 |
| label |
1-Aminoanthraquinone, α-Aminoanthraquinone, α-Aminoanthraquinone, 1-Amino-9,10-anthracenedione, Aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:82-45-1
MDL number:MFCD00001213
EINECS number:201-423-5
RTECS number:CB5075000
BRN number:396360
PubChem number:24890825
Physical property data
1. Properties: ruby red crystal.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 253~254
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined Determined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (kPa, 25ºC): Not determined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit ( %, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in ethanol, benzene, and chloroform , ether, glacial acetic acid, hot nitrobenzene and hydrochloric acid, almost insoluble in water. Slightly soluble in ethanol.
Toxicological data
1. Acute toxicity:
Rat abdominal LD50: 1500 mg/kg; Rat LDL0: 600 mg/kg;
Mouse caliber LD: >10 gm/ kg; rat abdominal cavity LD50: 6020mg/kg;
2. Oncogenicity: rat caliber TDL0: 2400 mg/kg/60W-I; rat caliber TD: 3000 mg/kg/60W-I ;
3. Teratogenicity: mice: 250 mg/kg
Ecological data
None
Molecular structure data
5. Molecular property data:
1. Molar refractive index: 62.90
2. Molar volume (cm3/mol): 161.3
3. Isotonic specific volume (90.2K): 462.7
4. Surface tension (dyne/cm): 67.6
5. Polarizability (10-24cm3): 24.93
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 5
6. Topological molecule polar surface area 60.2
7. Number of heavy atoms: 17
8. Surface charge: 0
9. Complexity: 352
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It is more toxic and irritating than anthraquinone. Can cause skin allergies and cause eczema. The production workshop should be well ventilated and the equipment should be sealed. Protective equipment should be worn during operation.
2. Avoid inhaling the dust of this product and avoid contact with eyes and skin.
Storage method
Store in a sealed, cool place away from light.
Packaged in iron drums lined with plastic bags. Net weight per barrel is 25kg or 50kg. Store in a cool, ventilated place. Moisture-proof and sun-proof. Store and transport according to regulations on toxic chemicals.
Synthesis method
1. Nitrification reduction method: Anthraquinone is nitrated with mixed acid to obtain nitroanthraquinone. The reaction product is filtered and washed with hot water until neutral, and then refined with sodium sulfite. Among them, 2-nitroanthraquinone and sodium sulfite generate anthraquinone-2-sodium sulfonate that is easily soluble in water, which is removed by filtering and washing. The obtained 1-nitroanthraquinone is reduced with sodium sulfide to obtain crude 1-aminoanthraquinone, which is refined by adding alkali solution and insurance powder to remove the diaminoanthraquinone, and then oxidized with oxygen, filtered, and dried to obtain the finished product.
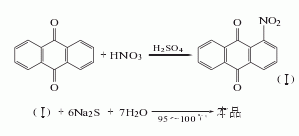
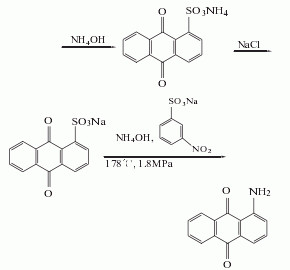
2. Anthraquinone sulfonate ammonolysis method: Anthraquinone reacts with fuming sulfuric acid in the presence of mercury sulfate to generate anthraquinone-1-sulfonic acid, which is neutralized with ammonia and then replaced with sodium salt, and then catalyzed by sodium m-nitrobenzene sulfonate Under ammonolysis, 1-aminoanthraquinone is generated, and the finished product is obtained after refining.
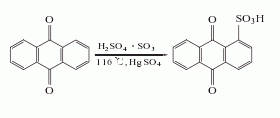
Purpose
1. Determine nitrite. Dyes and pharmaceutical intermediates. Organic Synthesis. 2. Important dye intermediates. It can be used to produce reduced khaki 2G, reduced red brown R, reduced olive green B, olive T, olive R, reduced gray M, dispersed red 3B, as well as reactive brilliant blue KN-R and reactive brilliant blue M-BR.
extended-reading:https://www.bdmaee.net/fascat4233-catalyst-butyl-tin-mercaptan-fascat-4233/extended-reading:https://www.bdmaee.net/nt-cat-bdmaee-catalyst-cas3033-62-3-newtopchem/extended-reading:https://www.bdmaee.net/nt-cat-ea-104-catalyst-cas10027-41-9-newtopchem/extended-reading:https://www.bdmaee.net/nt-cat-pc520-catalyst-cas10294-43-5-newtopchem/extended-reading:https://www.newtopchem.com/archives/979extended-reading:https://www.newtopchem.com/archives/category/products/page/87extended-reading:https://www.newtopchem.com/archives/44478extended-reading:https://www.bdmaee.net/dabco-33-lsi-dabco-33lsi/extended-reading:https://www.newtopchem.com/archives/40325extended-reading:https://www.newtopchem.com/archives/45212


